Diagnosis of Avian Metapneumovirus – Antigen determination
The following methods can be used to identify the virus:
Laboratory diagnosis of Avian Metapneumovirus can also be done by antibody determination.
Virus isolation
Because the aMPV virus is present in both turkeys and chickens for a short period after infection (3-5 days), virus isolation should be attempted using fresh material at the very first signs of disease. Upper respiratory tract material (sinus, nasal tissue, turbinate or nasal swabs) is more likely to yield virus than is tracheal tissue.
The use of sentinel birds or passaging the collected material in SPF birds may be helpful in this respect.
Viral isolation can be attempted in:
- embryonated specific pathogen free (SPF) eggs inoculated via the yolk sac.
- tracheal organ cultures (TOC).
- different cells cultures (for example chicken embryo fibroblasts) . Isolation from field material of types A and B cannot be done in cell culture; with these types TOC and embryo inoculation via the yolk sac are the only methods available. In The United States, it is reported that cell cultures can be used to isolate type C, but usually 2 yolk sac passages are given to field material before it is inoculated onto various different types of cell culture.
The virus causes haemorrhages in the embryos and some mortality; the egg fluids may be inoculated onto cell cultures (VERO, CEF) where CPE can be seen,often after several blind passages. Once isolated the virus can be adapted to grow in a variety of cell cultures (e.g. VERO, CEF, etc). When working with tracheal organ cultures (TOC) it must be kept in mind that subtype C is not ciliostatic, therefore it is not possible to use TOC’s for the isolation of this subtype from field material.
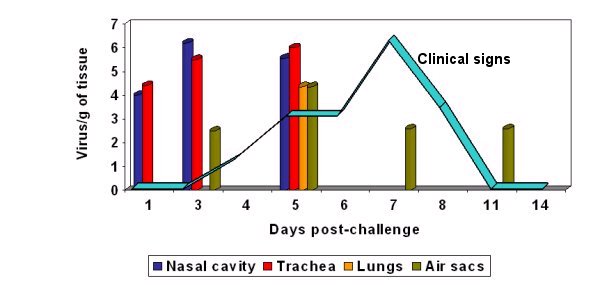
Graph 1: aMPV can be isolated for only a short period of time after infection. Once flocks are showing clinical signs of infection the virus can often no longer be isolated
Cook, J. K. A. (2000). Avian rhinotracheitis. In: Revue Scientifique et Technique, Office International des Epizooties, 19, pp 602-613.
Immunofluorescence Test
Fluorescein-conjugated antibodies that attach to the virus when present in a sample. The staining method known as immune histochemistry enables the recognition of the virus in the tissues and organs.
Immune histochemistry
Staining method that enables the recognition of the virus in tissues, infected TOC or cell culture.
Electron microscopy
Recognition of the virus morphology.
top
RT-PCR and RFLP
The RT-PCR (reverse transcriptase-polymerase chain reaction) is being increasingly used to detect and differentiate between strains of the virus. Dry swabs (at least 10/flock), taken from the oropharynx and choanal cleft, are normally collected for RT-PCR analysis. They can be transported to the laboratory at ambient temperature.
This technique is based on determining and comparing the sequencing of nucleotide fragments of certain genes of the virus. Primers are selected that may either detect all known types of the virus or that can specifically detect different types. It gives results very quickly, but it is important to remember that the sensitivity of the procedure and the durability of the DNA product may result in contaminations from previous PCRs carried out in the same laboratory and that subtype specific RT-PCR’s might be needed in order to differentiate all existing subtypes of the virus.
The PCR technology can also be used in surveys to detect the prevalence of different aMPV subtypes in the field.
top

